|
Size: 3424
Comment:
|
Size: 10471
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 1: | Line 1: |
| ## page was renamed from DryIce = Storing Excess Atmospheric CO2 as a liquid in the deep ocean = |
= Storing Excess Atmospheric CO₂ as a liquid in the deep ocean = |
| Line 4: | Line 3: |
| First, it would be better to stop burning carbon entirely. That said, we would burn a lot more carbon if we start wars trying to force other countries to stop. Server sky and space energy is the best alternative to burning carbon, but that isn't here yet. When we have sufficient energy from space, we can use some to clean up the atmosphere, so how can we do it? | First, it would be better to stop burning carbon entirely. That said, we would burn a lot more carbon if we start wars trying to force other countries to stop. Server sky and space energy is the best alternative to burning carbon, but that isn't here yet. When we have abundant energy from space, we can use some to clean up the atmosphere, so how can we do it? |
| Line 8: | Line 7: |
| Assume we are REALLY STUPID, and make 500ppm (by weight) of CO2 before we come to our senses. How much CO2 is that? | Assume we are REALLY STUPID, and make 500ppm (by weight) of excess CO₂ before we come to our senses. How much CO₂ is that? |
| Line 10: | Line 9: |
| The atmosphere weighs 15 pounds per square inch, or about 10,000 kilograms per square meter. 500ppm by weight CO2 would be 5 kilograms of CO2 per square meter, or 5000 tons per square kilometer, or 2.5 trillion tons for the entire earth. | The atmosphere weighs 15 pounds per square inch, or about 10,000 kilograms per square meter. 500ppm by weight CO₂ would be 5 kilograms of CO2 per square meter, or 5000 tons per square kilometer, or 2.5 trillion tons for the entire earth. |
| Line 12: | Line 11: |
| The deep ocean is about 4C; the pressure at 3km depth is about 300 bar. CO2 is a liquid at those pressures and temperatures and pressures, somewhat denser than water and more compressable. | The deep ocean is about 4C; the pressure at 3km depth is about 300 bar. CO₂ is a liquid at those pressures and temperatures and pressures, somewhat denser than water and more compressible. |
| Line 18: | Line 17: |
| ||<-2>Density vs Depth for CO2 and '''PURE'''(not salt) water; add about 15kg/m^3^ for the salt || ||<-2> {{attachment:dens.png| |width=800}} || |
||<-2>Density vs Depth for CO₂, H₂O, and salt water (guesstimated) || ||<-2> {{attachment:dens1.png| |width=800}} || ||<-2> CO₂ data: http://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Action=Page || ||<-2> H₂O data: http://webbook.nist.gov/cgi/fluid.cgi?ID=C7732185&Action=Page || ||<-2> seawater density guesstimated from H₂O + 25kg/m^3^ || |
| Line 21: | Line 23: |
| 2.5 trillion tons of Liquid CO2 occupies about 2400 cubic kilometers. | 2.5 trillion tons of liquid CO₂ occupies about 2400 cubic kilometers. |
| Line 23: | Line 25: |
| So, imagine lining a deep ocean rift or narrow depression, below 3000 meters depth, with an impermeable liner, and filling it with liquid CO2. Cover with a floating lid to keep the CO2 from dissolving in seawater. The ocean surface above this lid must be kept clear of shipping. We don't want one sinking and penetrating the lid. | So, imagine lining a deep ocean rift or narrow depression, below 3000 meters depth, with an impermeable liner, and filling it with liquid CO₂. Cover with a floating lid to keep the CO₂ from dissolving in seawater. The ocean surface above this lid must be kept clear of shipping. We don't want a ship sinking and penetrating the lid. |
| Line 25: | Line 27: |
| The area of the ocean is approximately 360 million square kilometers. Displacing 2400 cubic kilometers of ocean will raise sea levels by about 7 millimeters. | The area of the ocean is approximately 360 million square kilometers. Displacing 2400 cubic kilometers of ocean will raise sea levels by about 7 millimeters. |
| Line 27: | Line 29: |
| We capture CO2 out of the atmosphere (somehow), compress it isothermally to liquid (10MJ/ton? WAG) and push it down a pipe into the reservoir, using perhaps 3E19 joules to do the work. With 1TW devoted to the task, we could do all that sequestration in a year. In future years, we can release some if we need to stop an ice age. | If the liquid CO₂ goes into a dammed canyon, with 15 degree sloping sides and a 5 degree draw, then the area of the canyon will be large. The width to the side is 7.5 times the depth, and the length is 11.4 times the depth, so the volume is 14.2*depth^3^. The depth would be 5.5km, the length would be 63 km, and the width would be 41 km, occupying 1290 square kilometers of sea floor, or 3.5 parts per million of the ocean floor. Whether there is any suitable location (deep, steep edges, no unique species incapable of relocation) is an open question. |
| Line 29: | Line 31: |
| Most of the CO2 reservoir should be "geologically temporary" - a thousand years at most. Over time, the carbon should be separated and put to work elsewhere, as structure or biomass. | Given the relatively small density differences between seawater and LCO₂, perhaps 10% of the density difference across a water dam on the surface, the dam itself could be relatively steep. While building a concrete dam at that depth is probably impossible, a fill dam with reinforcements and 45 degree facings should be quite robust. That would consume 200 cubic kilometers of rock, perhaps mined from the future reservoir area. This would be a gargantuan reservoir, granted. But with rapidly increasing terawatts from space, we will have the resources to do this, as well as other tasks necessary to restore the Earth to a perpetually sustainable state. === Using the Romanche Trench? === The rift between the east and west ridges of the mid-Atlantic ridge is an interesting place for a LCO₂ reservoir, though maybe not deep enough. There are volcanoes on the ridge forming Iceland and the Azores, and hundreds of earthquakes per year along the length of the ridge, but with moment magnitudes typically less than 7.0, these will probably not damage a properly designed rock-fill dam, or the reservoir cover. There will be avalanches along the sides, so these will need to be fenced or channelled so they do not puncture the cover. {{attachment:Romanche1.png||width=800}} The [[http://en.wikipedia.org/wiki/Romanche_Trench | Romanche Trench ]] pictured above (with the last 35 years of earthquakes shown as dots) runs atop an east-west transform fault across the mid-Atlantic ridge. It is 7800 meters deep, 300km long, and 19km wide, with walls formed by the abyssal Atlantic deeps. Dams a few kilometers high may be possible. If two dams were 20 km wide, 1 km high in the middle across a presumably parabolic depression, and had a 30 degree slope on the faces, they would contain about 14km^3^ of rock. If they were 200 km apart, the reservoir between them would hold 2600 km^3^ of LCO₂. This would be a massive engineering project - for comparison, the [[ http://en.wikipedia.org/wiki/Three_Gorges_Dam | Three Gorges Dam ]] in China contains only 0.1 km^3^ of rock fill and 0.027km^3^ of concrete facing. But the rock could probably be dumped from surface barges, a simpler proposition than hauling it in by truck and placing it with laborers. With the surface of the LCO₂ at 6800 meters depth, the sea water density is perhaps 1050 kg/m^3^ and the LCO₂ density is perhaps 1150 kg/m^3^. A buoyant cap on the LCO₂ might be a plastic membrane a millimeter thick with a density of 1100 kg/m^3^, perhaps with an additional barrier of "balloons" to heal small breeches. The membrane will weigh 1100 tonnes per square kilometer, 4.4 million tonnes for the whole barrier. For comparison, global plastic production in 2010 was [[ http://www.businessvibes.com/blog/industry-insight-global-plastics-industry | 265 million tonnes ]]. === Wild Handwaving to be replaced with Improved Calculation === We capture CO2 out of the atmosphere (somehow), compress it isothermally to liquid (300MJ/ton? WAG) and push it down a pipe into the reservoir, using perhaps 1E21 joules to do the work. With 10TW devoted to the task, we could do all that sequestration in three years. In future years, we can release some if we need to stop an ice age. ## Eric Drexler uses 1E21J, I don't have his source. Most of the LCO₂ reservoir should be "geologically temporary" - two hundred years at most. Over time, the carbon should be separated and put to work elsewhere, as structure or biomass. |
| Line 33: | Line 58: |
| == Turning Back into Oxygen and Carbon == | === Turning Back into Oxygen and Carbon === |
| Line 35: | Line 60: |
| The heat of formation of CO2 is around 400KJ/mole, about 10MJ/Kg, about 20GJ per ton at 50% efficiency. At a 20TW rate, the process might take 80 years. | The heat of formation of CO₂ is around 400KJ/mole, about 10MJ/Kg, about 20GJ per ton at 50% efficiency. At a 20TW rate, the process might take 80 years. |
| Line 37: | Line 62: |
| == Launching to Space == | === Launching to Space === |
| Line 39: | Line 64: |
| As we produce habitats for life in space, we will need carbon and energy for them. One place to get it is from our under-ocean store. Launching will use a lot of energy per ton; If we launch with 50% efficiency to 10,000 m/s, then we will need 100GJ per ton, 10,000 times as much energy as we needed to sequester it. At a 20TW rate, the process might take 400 years. | As we produce habitats for life in space, we will need carbon and oxygen for agriculture. One place to get it is from our under-ocean store. Launching will use a lot of energy per ton; If we launch with 50% efficiency to 10,000 m/s, then we will need 100GJ per ton, 10,000 times as much energy as we needed to sequester it. At a 20TW rate, the process might take 400 years. But there is abundant oxygen on the moon - carbon, not so much. So if we only ship up the carbon and keep the oxygen down here, we can do so with less energy. We can start with 1 ton of CO₂, apply 20 GJ at 50% efficiency, and produce a 0.36 tons of elemental carbon. Perhaps we should just bury it again, but let's launch it instead. That needs 36 GJ . We were making CO₂ for habitat agriculture, so now let's go get some oxygen from the moon. At 50% efficiency (WAG, yet again), we can extract a ton of oxygen from !SiO₂ with about 57GJ. To make a ton of CO₂, we will need 727 kg of oxygen, or about 42 GJ of extraction energy. The lunar gravity well is shallow, so only 6GJ per ton gets the oxygen to the space habitat. Total energy: 20+36+42+6 or 104GJ. No savings. OTOH, if lunar energy was free, and we were only worried about the energy and heating costs on earth, then the earth energy component of this complex system is "only" 56GJ . Still not very attractive, compared to just launching dry ice and getting it over with, no lunar infrastructure needed. The delta V to bring carbon in from the outer solar system is daunting, and there isn't much solar energy out there to make the carbon or produce the delta V. Interesting idea, but let's use Earth CO₂ and cheap "local" energy first. |
| Line 41: | Line 70: |
=== Again, it would be best to avoid this === This CO₂ repository should not need to happen. Nobody should be building more carbon burners because something like this will relieve them of the responsibility. We should not be pumping the world's CO₂ up to 700ppm, and this rather massive geo-engineering project should only happen to save the planet from climate disaster, not to permit the continuation of sloppy practices. Storing CO₂ like this is only a temporary stopgap, while systems are built to eliminate the CO₂ and get back to 200ppm to 250ppm levels. If we decide to build a 100TW of systems to turn CO₂ back into carbon and oxygen in 20 years, that might be preferable. Our responsibility is for the well-being of the planet, not for picking culprits and punishing them. If you are a live human being reading this, you are drawing energy from the planet, and you are one of the culprits. Best to develop perpetually sustainable ways to feed your own energy habits, not punish others for failing to meet standards you yourself cannot. Nature is not healed by misanthropy. ----- BTW, ocean sequestration of CO₂ is not a new idea; see for example: . [[ http://www.netl.doe.gov/publications/factsheets/project/Proj237.pdf ]] Some studies seem to suggest mixing CO₂ with seawater, which is stupid. That might kill the ocean as we know it, resulting in an anoxic ocean and vast quantities of hydrogen sulfide released into the atmosphere, killing almost everything on land (See Peter Ward's "Under A Green Sky"). We should not try to disperse CO₂, but store it until we develop benign energy sources to launch it or process it back into carbon and oxygen. Until then, ride a bike and walk, reduce your consumption, and use low power computing. Please. |
Storing Excess Atmospheric CO₂ as a liquid in the deep ocean
First, it would be better to stop burning carbon entirely. That said, we would burn a lot more carbon if we start wars trying to force other countries to stop. Server sky and space energy is the best alternative to burning carbon, but that isn't here yet. When we have abundant energy from space, we can use some to clean up the atmosphere, so how can we do it?
Assume we are REALLY STUPID, and make 500ppm (by weight) of excess CO₂ before we come to our senses. How much CO₂ is that?
The atmosphere weighs 15 pounds per square inch, or about 10,000 kilograms per square meter. 500ppm by weight CO₂ would be 5 kilograms of CO2 per square meter, or 5000 tons per square kilometer, or 2.5 trillion tons for the entire earth.
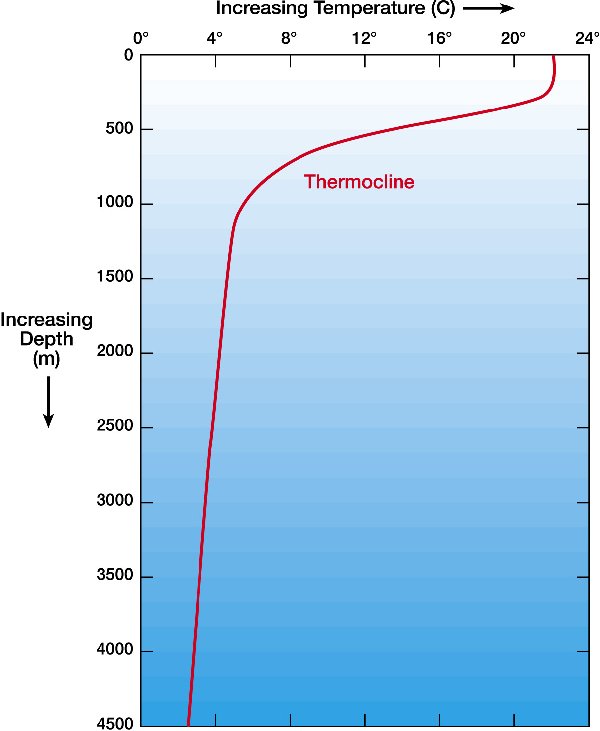
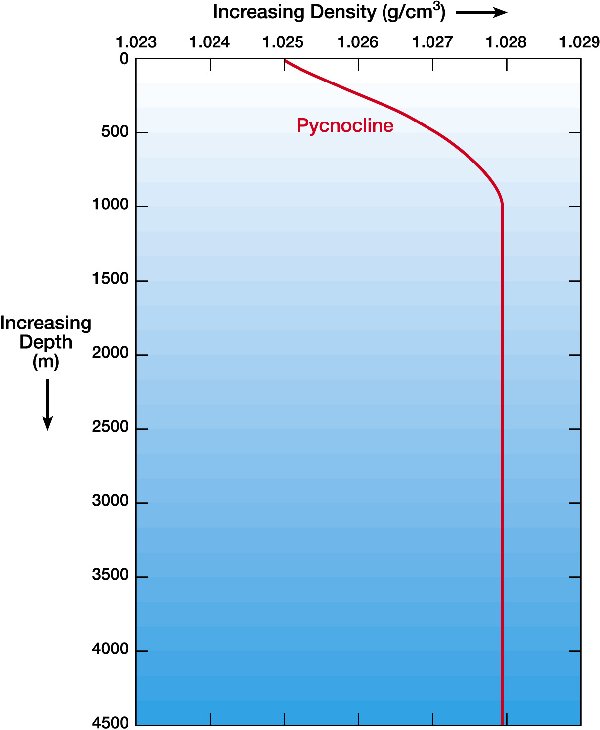
The deep ocean is about 4C; the pressure at 3km depth is about 300 bar. CO₂ is a liquid at those pressures and temperatures and pressures, somewhat denser than water and more compressible.
Temperature versus Depth |
Density versus Depth |
|
|
from http://windows2universe.org note:curves are for SALT WATER |
|
|
|
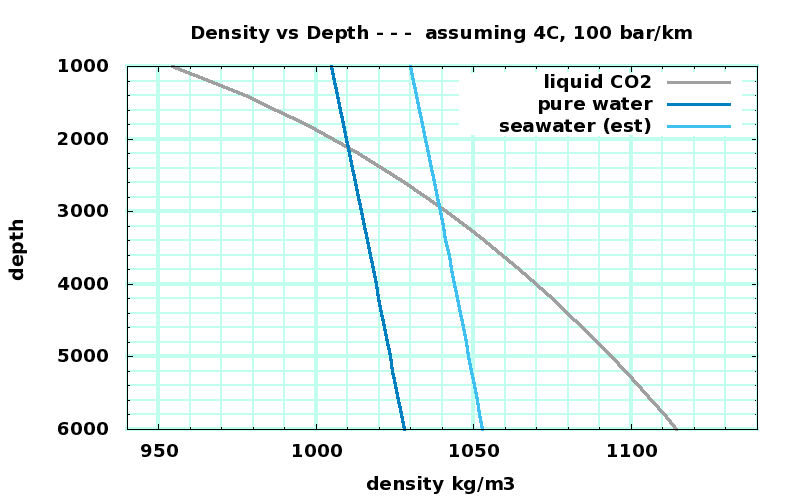
Density vs Depth for CO₂, H₂O, and salt water (guesstimated) |
|
|
|
CO₂ data: http://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Action=Page |
|
H₂O data: http://webbook.nist.gov/cgi/fluid.cgi?ID=C7732185&Action=Page |
|
seawater density guesstimated from H₂O + 25kg/m3 |
|
2.5 trillion tons of liquid CO₂ occupies about 2400 cubic kilometers.
So, imagine lining a deep ocean rift or narrow depression, below 3000 meters depth, with an impermeable liner, and filling it with liquid CO₂. Cover with a floating lid to keep the CO₂ from dissolving in seawater. The ocean surface above this lid must be kept clear of shipping. We don't want a ship sinking and penetrating the lid.
The area of the ocean is approximately 360 million square kilometers. Displacing 2400 cubic kilometers of ocean will raise sea levels by about 7 millimeters.
If the liquid CO₂ goes into a dammed canyon, with 15 degree sloping sides and a 5 degree draw, then the area of the canyon will be large. The width to the side is 7.5 times the depth, and the length is 11.4 times the depth, so the volume is 14.2*depth3. The depth would be 5.5km, the length would be 63 km, and the width would be 41 km, occupying 1290 square kilometers of sea floor, or 3.5 parts per million of the ocean floor. Whether there is any suitable location (deep, steep edges, no unique species incapable of relocation) is an open question.
Given the relatively small density differences between seawater and LCO₂, perhaps 10% of the density difference across a water dam on the surface, the dam itself could be relatively steep. While building a concrete dam at that depth is probably impossible, a fill dam with reinforcements and 45 degree facings should be quite robust. That would consume 200 cubic kilometers of rock, perhaps mined from the future reservoir area.
This would be a gargantuan reservoir, granted. But with rapidly increasing terawatts from space, we will have the resources to do this, as well as other tasks necessary to restore the Earth to a perpetually sustainable state.
Using the Romanche Trench?
The rift between the east and west ridges of the mid-Atlantic ridge is an interesting place for a LCO₂ reservoir, though maybe not deep enough. There are volcanoes on the ridge forming Iceland and the Azores, and hundreds of earthquakes per year along the length of the ridge, but with moment magnitudes typically less than 7.0, these will probably not damage a properly designed rock-fill dam, or the reservoir cover. There will be avalanches along the sides, so these will need to be fenced or channelled so they do not puncture the cover.
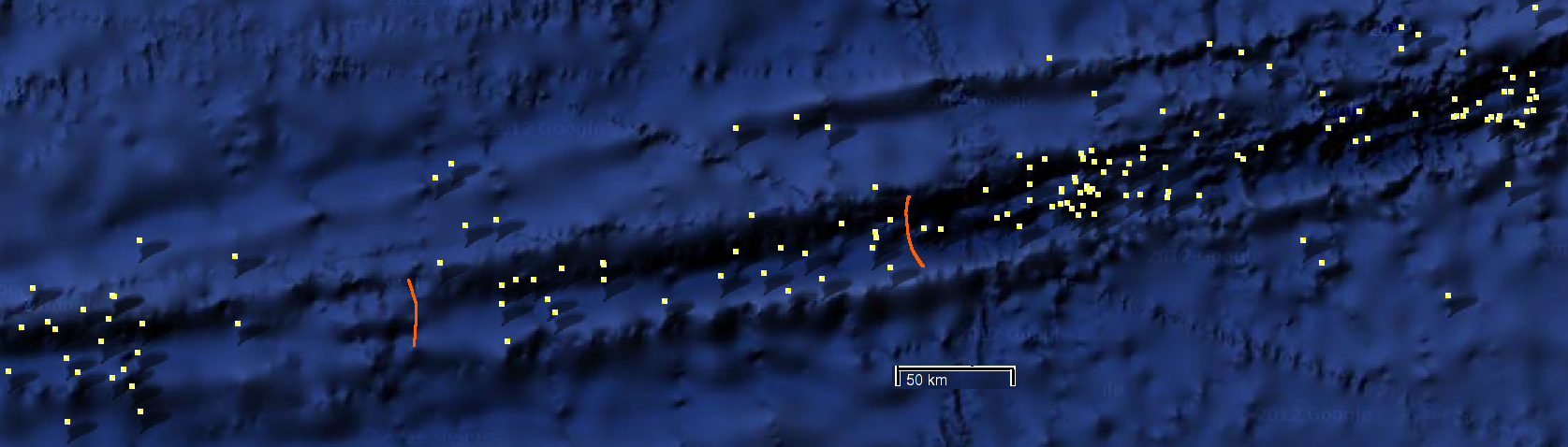
The Romanche Trench pictured above (with the last 35 years of earthquakes shown as dots) runs atop an east-west transform fault across the mid-Atlantic ridge. It is 7800 meters deep, 300km long, and 19km wide, with walls formed by the abyssal Atlantic deeps. Dams a few kilometers high may be possible.
If two dams were 20 km wide, 1 km high in the middle across a presumably parabolic depression, and had a 30 degree slope on the faces, they would contain about 14km3 of rock. If they were 200 km apart, the reservoir between them would hold 2600 km3 of LCO₂. This would be a massive engineering project - for comparison, the Three Gorges Dam in China contains only 0.1 km3 of rock fill and 0.027km3 of concrete facing. But the rock could probably be dumped from surface barges, a simpler proposition than hauling it in by truck and placing it with laborers.
With the surface of the LCO₂ at 6800 meters depth, the sea water density is perhaps 1050 kg/m3 and the LCO₂ density is perhaps 1150 kg/m3. A buoyant cap on the LCO₂ might be a plastic membrane a millimeter thick with a density of 1100 kg/m3, perhaps with an additional barrier of "balloons" to heal small breeches. The membrane will weigh 1100 tonnes per square kilometer, 4.4 million tonnes for the whole barrier. For comparison, global plastic production in 2010 was 265 million tonnes.
Wild Handwaving to be replaced with Improved Calculation
We capture CO2 out of the atmosphere (somehow), compress it isothermally to liquid (300MJ/ton? WAG) and push it down a pipe into the reservoir, using perhaps 1E21 joules to do the work. With 10TW devoted to the task, we could do all that sequestration in three years. In future years, we can release some if we need to stop an ice age.
Most of the LCO₂ reservoir should be "geologically temporary" - two hundred years at most. Over time, the carbon should be separated and put to work elsewhere, as structure or biomass.
MORE DETAILS on process and energy requirements later.
Turning Back into Oxygen and Carbon
The heat of formation of CO₂ is around 400KJ/mole, about 10MJ/Kg, about 20GJ per ton at 50% efficiency. At a 20TW rate, the process might take 80 years.
Launching to Space
As we produce habitats for life in space, we will need carbon and oxygen for agriculture. One place to get it is from our under-ocean store. Launching will use a lot of energy per ton; If we launch with 50% efficiency to 10,000 m/s, then we will need 100GJ per ton, 10,000 times as much energy as we needed to sequester it. At a 20TW rate, the process might take 400 years.
But there is abundant oxygen on the moon - carbon, not so much. So if we only ship up the carbon and keep the oxygen down here, we can do so with less energy. We can start with 1 ton of CO₂, apply 20 GJ at 50% efficiency, and produce a 0.36 tons of elemental carbon. Perhaps we should just bury it again, but let's launch it instead. That needs 36 GJ . We were making CO₂ for habitat agriculture, so now let's go get some oxygen from the moon. At 50% efficiency (WAG, yet again), we can extract a ton of oxygen from SiO₂ with about 57GJ. To make a ton of CO₂, we will need 727 kg of oxygen, or about 42 GJ of extraction energy. The lunar gravity well is shallow, so only 6GJ per ton gets the oxygen to the space habitat. Total energy: 20+36+42+6 or 104GJ. No savings. OTOH, if lunar energy was free, and we were only worried about the energy and heating costs on earth, then the earth energy component of this complex system is "only" 56GJ . Still not very attractive, compared to just launching dry ice and getting it over with, no lunar infrastructure needed.
The delta V to bring carbon in from the outer solar system is daunting, and there isn't much solar energy out there to make the carbon or produce the delta V. Interesting idea, but let's use Earth CO₂ and cheap "local" energy first.
Again, it would be best to avoid this
This CO₂ repository should not need to happen. Nobody should be building more carbon burners because something like this will relieve them of the responsibility. We should not be pumping the world's CO₂ up to 700ppm, and this rather massive geo-engineering project should only happen to save the planet from climate disaster, not to permit the continuation of sloppy practices.
Storing CO₂ like this is only a temporary stopgap, while systems are built to eliminate the CO₂ and get back to 200ppm to 250ppm levels. If we decide to build a 100TW of systems to turn CO₂ back into carbon and oxygen in 20 years, that might be preferable.
Our responsibility is for the well-being of the planet, not for picking culprits and punishing them. If you are a live human being reading this, you are drawing energy from the planet, and you are one of the culprits. Best to develop perpetually sustainable ways to feed your own energy habits, not punish others for failing to meet standards you yourself cannot. Nature is not healed by misanthropy.
BTW, ocean sequestration of CO₂ is not a new idea; see for example:
Some studies seem to suggest mixing CO₂ with seawater, which is stupid. That might kill the ocean as we know it, resulting in an anoxic ocean and vast quantities of hydrogen sulfide released into the atmosphere, killing almost everything on land (See Peter Ward's "Under A Green Sky"). We should not try to disperse CO₂, but store it until we develop benign energy sources to launch it or process it back into carbon and oxygen. Until then, ride a bike and walk, reduce your consumption, and use low power computing. Please.