Electrochromic Light Shutters
|
Electrochromic materials undergo pigment changes and color shifts by redox reactions in an electrochemical cell. By applying a small voltage and current, these cells can switch from transparent to pigmented (i.e. white or black). There are a wide range of materials and behaviors. |
Varieties of Electrochromic Behavior
White to Clear over Transparent Substrate
Adjusting reflectivity changes light pressure. Changing from transparent to a perfect Lambertian white reflector changes the on-axis light pressure from 0 to ( 1 + 1/π ) ( P/c ). Off axis sideways thrust is proportional to sin( 2 θ ) , with a contribution from incident light but none from the reflective light.
Black to Clear over Reflector and Opaque Substrate (the plan)
If the area just behind the electrochromic material is a shiny reflector (similar to electronic car mirrors), we can switch from blackish near-zero-albedo to a mirror. Tilting the mirror by provides sideways thrust. Sun-direction light pressure at black will be somewhat less than P/c; the electrochromic material may remain transparent in some bands. With a clear window exposing the mirror underneath, sun-direction thrust is < 2(P/c)cos(Θ)cos(Θ) = 2(P/c)cos²(Θ) and the sideways thrust is 2(P/c)cos(Θ)sin(0) = (P/c)sin(2Θ).
If the substrate is reflective/conductive - say aluminum and molybdenum over an insulator - this permits two important things:
- 1) We can cut slot antennas into it. That will reduce the area of the thrusters a little, but it will increase the radio "dish" area.
- 2) We can put thrusters on both sides, and have different effects on the sides. This is most important for a thinsat recovering from a yaw or pitch spin (perhaps caused by a collision); electrochromics are slow (seconds or more), and if light can shine through, we cannot change front/asymmetry fast enough to work against the spin. OTOH, we can make sure that the edge approaching the sun is reflective and the receding edge is black - that will slow down the spin, and the electrochromics are still fast enough to slowly shift with precession. Roll spins will be harder, but we can use reflected light pressure on a pitched thinsat to work against the roll; this will precess faster if the pitch is large, so we can only use small pitches and remove spin gradually.
Switchable Mirror Transition Metal Hydrides over transparent substrate (the most thrust)
Transition metal electrochromics can shift all the way from transparent to a mirror. The light pressure is 50% higher, and the off-axis sideways thrust from the mirror doubles.
Here is an Lawrence Berkeley Lab video of a transition metal electrochromic window in action. I do not know how close this material is to deployment, but more traditional electrochromics are still usable for server sky.
These are more speculative - I haven't seen much work over the past few years, and some early switchable mirrors seem to be switched by hydrogen gas. That is way too complicated for thinsats, and hydrogen will leak through the very thin coverglass.
Further, switching all the way to transparent precludes the use thruster area for radio antennas, and clear thrusters are less useful for high speed spin reduction.
Transparent Electrodes
An expensive component of electrochromic devices (and solar cells) is the Indium Tin Oxide (ITO) transparent electrode. Billions of square meters of electrode can use millions of tons of indium; it may be possible to use lensing and strips to slightly focus the light away from narrow, opaque metal electrodes, allowing ultrathin, high sheet resistance ITO electrodes, which conduct laterally only a few micrometers. While this would be unacceptable for human-visible displays and windows, it would benefit solar cells and light shutters, especially combined with reflection-reducing surface treatments. The solar light source is collimated to half a degree, and we can take advantage of this.
We may also find other transparent electrode materials, such as Aluminum Zinc Oxide (AZO) , which are earth (and probably lunar ) abundant. On earth, aluminum is 40,000 times more abundant than tin, and zinc is 1500 times more abundant than indium. On the moon, zinc will not be hydrologically beneficiated into ore bodies like it is on earth - it may turn out that asteroidal indium may be easier to extract. Most likely, for the next century, we will continue to ship zinc from earth, even as we extract many other thinsat materials from lunar regolith.
Notes from Electrochromism and Electrochromic Devices
Monk, Mortimer, and Rosseinsky, Cambridge University Press, 2007
- Paul M. S. Monk - Manchester Metropolitan University
- Redox reaction:
- reduction == gains an electron (for example from a hydrogen)
- oxidation == loses an electron (for example, to an oxygen completing its outer shell)
Few mentions of thickness. Electrode thicknesses of 3 to 4μm thick are mentioned, and monolayers of EC material
- Type-I electrochromes are soluble and stay in solution
- example: aqueous methyl viologen ( 1,1'-dimethyl-4-4'-bipyridilium ), reduction from colorless to intense blue
- example: phenothiazines such as Methylene Blue in non-aqueous solutions
- Gentex uses a combination of a phenothiazine and viologen for switchable car mirrors. These "self-erase" to clear
- Gentex uses the 787 dreamliner window shutters
- Type-II electrochromes are soluble in their colorless forms but precipitate to a colored solid on the surface of the electrode
- example, cyanophenyl paraquat in aqueous solution
- Type-III electrochromes remain solid at all times
- most inorganic electrochromes, such as metal oxides including Mo, Ni, Ti and W. WO₃ is most common
Coloration efficiency: log(Io/I)/Q, 20 to 120 colombs/cm2
- probably most compatable with server sky space environment (WAG KHL)
- Contrast ratio: Lambert white diffuse over Lambert colored diffuse at a specific wavelength.
- 3 is barely visible, 25:1 is typical type-II, 10:1 for WO₃ | NiO
Electrostriction - electrochomics expands by 6% on reductive ion insertion.
- THIS COULD CURL THE THRUSTERS, depending on EC material thickness
- FTO - Fluorine doped tin oxide, like ITO indium tin oxide
- sol - colloidal suspension of small solid particles in a liquie
p139 amorphous films faster and superior coloration ν polycrystalline more chemically durable
The 1987 Donnelly Corp Lynam+Seah Solid State Mirror Patent
This is a solid state rear view mirror patent. There are probably better systems out there in 2013, but the patent has expired and this well written patent shows one way to do it. There are two electrochromic layers -
The mirror stack:
- 0) 1 to 3 mm front surface glass.
1) First conductive layer, ITO, TO, or CdSn. Transmission maximized by using a half or full wavelength at refractive index n=1.7 for ITO, hence 160nm works well for 550nm light. The resulting sheet resistance is 10-15 Ω/■ .
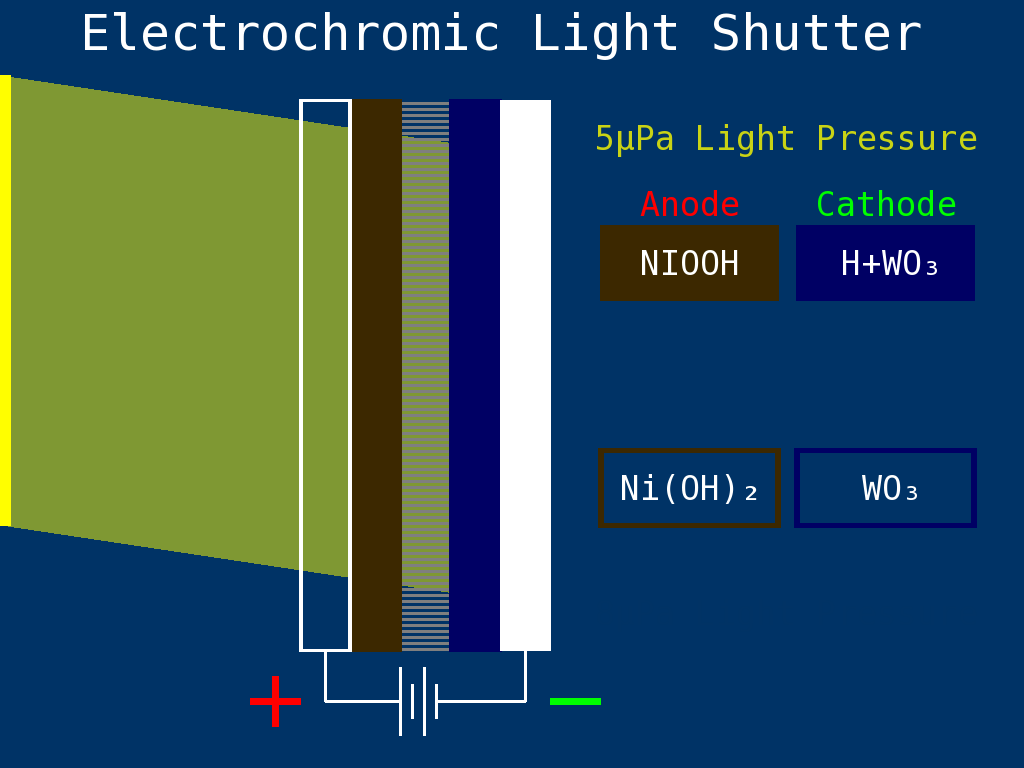
- 2) 400nm Nickel Hydroxide Ni(OH)₂ . Anodic electrochromic material, colors brown when electrically connected to a positive voltage, shedding an electron to the wire and an H⁺ proton into the electrolytic layer, becoming NiOOH.
3) 130nm Electrolytic layer (dry?), non-conductive but permeable to hydrogen ions. Ta₂O₅ used, but SiO₂ and Al₂O₃ may also work.
- 4) 80nm Tungsten Oxide WO₃ . Cathodic electrochromic material, colors blue when electrically connected to a negative voltage, absorbing an electron from the wire and an H⁺ proton from the electrolytic layer, becoming HWO₃
- 5) 100nm Conductive aluminum layer, the mirror. 28.2nΩ-m becomes 0.3Ω/■ .
Assume a similar stack, with 10μm glass over a 30μm aluminum substrate over 10μm glass (shutters on both sides):
# |
material |
thickness |
density |
g/m² |
resistivity |
resistance |
$/kg |
$/m² WAG |
2 |
surface glass |
10.0μm |
2.65 |
53.00 |
|
|
10.0 |
0.5300 |
2 |
ITO |
0.16μm |
7.16 |
2.28 |
|
15Ω/■ |
200 |
0.5600 |
2 |
NI(OH)₂ |
0.40μm |
4.10 |
3.28 |
|
|
2.00 |
0.0033 |
2 |
porous silica |
0.13μm |
2.00 |
0.52 |
|
|
2.00 |
0.0010 |
2 |
WO₃ |
0.08μm |
7.16 |
1.14 |
|
|
5.00 |
0.0057 |
1 |
Aluminum |
30.0μm |
2.70 |
81.00 |
28.2nΩ-m |
1mΩ/■ |
5.00 |
0.4050 |
|
Total |
51.5μm |
|
142.0 |
|
|
|
1.50 |
From the Lynam+Seah patent:
The preferred embodiment of the present invention is an electrochromic mirror comprising a plurality of solid state thin films deposited onto a single piece of glass 11 (FIG. 1). Neither the thickness nor type of glass used is critical. A typical glass substrate will be conventional soda glass at a thickness of from about one millimeter to about three millimeters. Use of a single piece of glass 11 greatly eases shatterproofing and convex fabrication problems discussed above.
The first layer deposited on glass substrate 11 is conductive layer 1. Conductive layer 1 can be of any conventional electroconductive coating, such as indium-tin oxide, tin oxide or cadmium stannate. The thickness of this layer is not critical, so long as it adequately conducts electricity across its entire surface. Also, its thickness should be selected so that the transmission of light through the electroconductive layer is maximized. In this regard, it is convenient to work with half wavelength or full wavelength optical thicknesses of the median wavelength for visible light (5500 angstroms). Optical thickness is physical thickness multiplied by the index of refraction of the material. In the case of indium-tin oxide, the index of refraction is about 1.7. Hence, an indium-tin oxide layer preferably has a thickness of approximately 1600 angstroms. This is about one-half wavelength in optical thickness (1600 conductivity (sheet resistance typically 10-15 ohms/square) across its entire surface and yet is sufficiently thin that it does not excessively darken the mirror.
The second layer applied is a nickel hydroxide electrochromic layer 2. Nickel hydroxide is an anodic electrochromic material in that it colors when electrically connected to a positive electrode. The thickness of the nickel hydroxide layer is critical. If it is too thick, the mirror will have low reflectivity. The nickel hydroxide layer will give the mirror a constant brownish cast. The mirror will be too dark for normal daylight use. Further, the mirror will be too slow in changing from daylight mode to night mode and visa versa.
On the other hand if the nickel hydroxide layer is too thin, the mirror will not darken sufficiently during nighttime conditions as a following car approaches. We have found that the nickel hydroxide layer must be from about 300 to about 600 angstroms thick. Most preferably, the nickel hydroxide layer is 400 angstroms thick.
The term "nickel hydroxide" as used herein must of course be understood to encompass variations from stoichiometrically pure Ni(OH).sub.2. Those skilled in the art will appreciate that the starting material for applying the nickel hydroxide layer is nickel oxide which, as explained below, is then hydrated to nickel hyd
roxide. It is not known whether such hydration ever becomes stoichiometrically complete.
The next layer applied to the mirror stack is a solid electrolytic layer 3. This layer serves to isolate the anodic and cathodic electrochromic layers. As such, it must be ion conducting, electron insulating, clear and must remain clear during bleaching of the electrochromic layers. Suitable materials include tantalum pentoxide, cesium oxide, aluminum oxide, magnesium fluoride, silicon dioxide and mixtures thereof. Tantalum pentoxide is the most preferred material. The thickness of this layer is also critical. If too thick, the mirror does not shift between daylight and night modes rapidly enough. If this layer is too thin, the mirror will have too great a tendency to discharge from its colored to an uncolored state. We have found that the solid electrolyte layer must have a thickness of from about 1000 to about 1500 angstroms, and most preferably 1300 angstroms.
Electrochromic layer 4 is a cathodic electrochromic material comprising tungsten oxide. Cathodic electrochromic materials are those which color when connected to a negative electrode. Thus when the mirror stack is subjected to an appropriate potential difference, both the nickel hydroxide and tungsten oxide layers will color. When that potential is reversed, both layers will clear.
The thickness of the tungsten oxide layer is also critical. If too thick, the mirror will respond too slowly to changes in light and will always be too dark. Daylight reflectivity will not be sufficient. If the layer is too thin, the mirror will not color sufficiently for nighttime conditions. We have found that the tungsten oxide layer should be from about 600 to about 1200 angstroms, and most preferably 800 angstroms.
Finally, an aluminum conductive and reflective layer 5 is applied. It is important only that this layer be sufficiently thick that it conducts readily over its entire surface and cannot be easily damaged. The aluminum layer serves both to reflect incident light and to conduct electricity to the tungsten oxide electrochromic layer. We have found that a thickness of from about 500 to about 1500 angstroms is adequate. The most preferable aluminum layer thickness appears to be about 1000 angstroms.
It is preferable that the nickel hydroxide layer be deposited ahead of the tungsten oxide layer in order of exposure to incident light. If deposited in that order, the mirror of the present invention will, in its uncolored state, reflect in excess of 70% of the incident light. In the colored state, it will reflect less than 10% of the incident light.
In contrast if the tungsten oxide layer were deposited in front of the nickel hydroxide layer in order of exposure to incident light, the mirror, with the preferred layer thicknesses will reflect no more than about 60% of the incident light in its uncolored state. Altering layer thicknesses to increase reflectivity in the uncolored state results in the mirror reflecting too much light in its colored state.
The half reactions which lead to the coloring and bleaching of the mirror stack are illustrated below: ##STR1##
The liberated election is drawn through the power circuit to which the two electrodes are connected. The liberated hydrogen ion migrates through the ion permeable, solid electrolyte layer.
The various layers 1-5 in the mirror stack of the present invention can be deposited in any conventional manner. Different deposition methods can be employed for different layers. Such techniques are well-known to those skilled in the art for all of the materials used in the present invention.
Common modes of fabrication of the electrochromically active layers 2 and 4, and electrolyte insulating layer 3, include evaporation and sputter deposition. The starting materials include high purity tungsten oxide, nickel oxide and tantalum pentoxide. The availability of moisture during the manufacturing process is critical to ultimate device performance in order to facilitate conversion of the nickel oxide to nickel hydroxide. We find it beneficial to fabricate the mirrors in an environment of at least 30% relative humidity. We find it beneficial to purposely introduce controlled amounts of moisture into the vacuum chamber during deposition of the tungsten oxide, tantalum pentoxide and nickel oxide layers.
We also find it beneficial to operate the vacuum deposition chamber at as high a pressure as can be tolerated in order to render layers 2, 3 and 4 somewhat porous in order that ion injection, ejection and transmission is rapid. For example in evaporation coating, we find that backfilling the deposition chamber to a partial pressure of about 10.sup.-4 torr sufficiently reduces mean free path that the desired porous coatings are obtained. EXPERIMENTAL RESULTS
Mirrors made in accordance with the present invention were tested for speed of response to changes in potential, reflectivity at potential extremes and durability through repeated cycling. The mirror in its uncolored state was subjected to a potential difference and the change in reflectivity was charted. In one second, the mirror was discharged and again, the change in reflectivity charted. A typical chart readout is illustrated in FIG. 2.
The foregoing cycle was repeated over 100,000 times. It was found that the mirror made in accordance with the present invention cycled from a reflectivity of in excess of 70% to a reflectivity of less than 10% in one to three seconds. This pattern continued through all 100,000 plus cycles.
Of course, it is understood that the foregoing is merely a preferred embodiment of the invention and that various changes and alterations can be made without departing from the spirit and broader aspects thereof.